Curium(III) chloride
In this article we are going to address the topic of Curium(III) chloride, which has sparked great interest and debate in different areas. Curium(III) chloride is a relevant topic that impacts people's daily lives, both on a personal and global level. Over the years, Curium(III) chloride has evolved and generated diverse perspectives and opinions, sparking endless discussions and analyzes about its importance, implications, and possible solutions. Therefore, it is essential to delve deeper and understand the complexity of Curium(III) chloride in order to form an informed opinion and contribute to the dialogue on this topic. In this article, we will explore different aspects of Curium(III) chloride and analyze its impact on today's society.
 | |
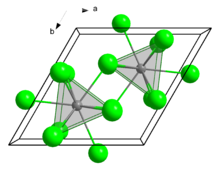 Crystal structure
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| Cl3Cm | |
| Molar mass | 353 g·mol−1 |
| Appearance | White solid (anhydrous) Light green solid (hydrate) |
| Melting point | 695 °C (1,283 °F; 968 K)[citation needed] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Curium(III) chloride is the chemical compound with the formula CmCl3.
Structure
Curium(III) chloride has a 9 coordinate tricapped trigonal prismatic geometry.[1]
Synthesis
Curium(III) chloride can be obtained from the reaction of hydrogen chloride gas with curium dioxide, curium(III) oxide, or curium(III) oxychloride at a temperature of 400-600 °C:
- CmOCl + 2HCl → CmCl3 + H2O
It can also be obtained from the dissolution of metallic curium in dilute hydrochloric acid:[2]
- 2Cm + 6HCl → 2CmCl3 + 3H2
This method has a number of disadvantages associated with the ongoing processes of hydrolysis and hydration of the resulting compound in an aqueous solution, making it problematic to obtain a pure product using this reaction.
It can be obtained from the reaction of curium nitride with cadmium chloride:[3]
- 2 CmN + 3 CdCl2 → 2 CmCl3 + Cd3N2
References
- ^ Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Butterworth, UK. p. 1270.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Wallmann, J. C.; Fuger, J.; Peterson, J. R.; Green, J. L. (1 November 1967). "Crystal structure and lattice parameters of curium trichloride". Journal of Inorganic and Nuclear Chemistry. 29 (11): 2745–2751. doi:10.1016/0022-1902(67)80013-7. ISSN 0022-1902. S2CID 97334114. Retrieved 3 July 2023.
- ^ Hayashi, Hirokazu; Takano, Masahide; Otobe, Haruyoshi; Koyama, Tadafumi (July 2013). "Syntheses and thermal analyses of curium trichloride". Journal of Radioanalytical and Nuclear Chemistry. 297 (1): 139–144. Bibcode:2013JRNC..297..139H. doi:10.1007/s10967-012-2413-7. S2CID 95792512.