MMAI
Throughout history, MMAI has been a topic of great interest and controversy. Since its inception, MMAI has captured the attention of academics, scientists, artists and the curious in general. Its impact on society and people's daily lives is undeniable, generating constant debates and reflections. In this article, we will explore different aspects and perspectives related to MMAI, analyzing its influence in different fields and its evolution over time. Additionally, we will examine how MMAI continues to be relevant today and how it will continue to make its mark in the future.
 | |
| Clinical data | |
|---|---|
| Other names | MMAI; MMAi; 5-Methoxy-6-methyl-2-aminoindan |
| Routes of administration | By mouth |
| Drug class | Selective serotonin releasing agent; Entactogen |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H15NO |
| Molar mass | 177.247 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
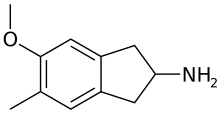
5-Methoxy-6-methyl-2-aminoindane (MMAI) is a drug of the 2-aminoindane group developed in the 1990s by a team led by David E. Nichols at Purdue University.[1] It acts as a less neurotoxic and highly selective serotonin releasing agent (SSRA) and produces entactogenic effects in humans.[1][2][3][4] It has been sold as a designer drug and research chemical online since 2010.
The drug is one of the only known monoamine releasing agents (MRAs) with greater than 100-fold selectivity for the serotonin transporter (SERT) over the dopamine transporter (DAT).[5]
MMAI has been shown to relieve stress-induced depression in rats more robustly than sertraline,[6] and as a result it has been suggested that SSRAs like MMAI and 4-methylthioamphetamine (4-MTA) could be developed as novel antidepressants with a faster onset of therapeutic action and superior effectiveness to current antidepressants such as the selective serotonin reuptake inhibitors (SSRIs).[7]
MMAI alone does not appear to produce serotonergic neurotoxicity with either acute or chronic administration in animals.[8][9] However, subsequent research found that a single high dose of MMAI could produce significant serotonergic neurotoxicity.[8][9] In addition, combination of MMAI with the dopamine releasing agent dextroamphetamine has been found to produce dose-dependent serotonergic neurotoxicity in animals.[8] Hence, MMAI is not a fully non-neurotoxic MDMA analogue.[8][9]
MMAI is the 2-aminoindane analogue of 3-methoxy-4-methylamphetamine (MMA).[10][3]
| Compound | Monoamine release (EC50, nM) | Ref | ||
|---|---|---|---|---|
| Serotonin | Norepinephrine | Dopamine | ||
| 2-AI | >10,000 | 86 | 439 | [11] |
| MDAI | 114 | 117 | 1,334 | [11] |
| MMAI | 31 | 3,101 | >10,000 | [11] |
| MEAI | 134 | 861 | 2,646 | [11] |
| d-Amphetamine | 698–1,765 | 6.6–7.2 | 5.8–24.8 | [12][13][14][15][16] |
| MDA | 160–162 | 47–108 | 106–190 | [17][14][18] |
| MDMA | 50–85 | 54–110 | 51–278 | [12][19][20][17][18] |
| 3-MA | ND | 58.0 | 103 | [14] |
| Notes: The smaller the value, the more strongly the compound produces the effect. The assays were done in rat brain synaptosomes and human potencies may be different. See also Monoamine releasing agent § Activity profiles for a larger table with more compounds. Refs: [11] | ||||
References
- ^ a b Marona-Lewicka D, Nichols DE (June 1994). "Behavioral effects of the highly selective serotonin releasing agent 5-methoxy-6-methyl-2-aminoindan". European Journal of Pharmacology. 258 (1–2): 1–13. CiteSeerX 10.1.1.688.1895. doi:10.1016/0014-2999(94)90051-5. PMID 7925587.
- ^ Li Q, Murakami I, Stall S, Levy AD, Brownfield MS, Nichols DE, Van de Kar LD (December 1996). "Neuroendocrine pharmacology of three serotonin releasers: 1-(1,3-benzodioxol-5-yl)-2-(methylamino)butane (MBDB), 5-methoxy-6-methyl-2-aminoindan (MMAi) and p-methylthioamphetamine (MTA)". The Journal of Pharmacology and Experimental Therapeutics. 279 (3): 1261–1267. doi:10.1016/S0022-3565(25)21285-X. PMID 8968349.
- ^ a b Rudnick G, Wall SC (February 1993). "Non-neurotoxic amphetamine derivatives release serotonin through serotonin transporters". Molecular Pharmacology. 43 (2): 271–276. doi:10.1016/S0026-895X(25)13609-2. PMID 8429828.
- ^ Luethi D, Kolaczynska KE, Docci L, Krähenbühl S, Hoener MC, Liechti ME (May 2018). "Pharmacological profile of mephedrone analogs and related new psychoactive substances" (PDF). Neuropharmacology. 134 (Pt A): 4–12. doi:10.1016/j.neuropharm.2017.07.026. PMID 28755886. S2CID 28786127.
- ^ Rudin D, Liechti ME, Luethi D (September 2021). "Molecular and clinical aspects of potential neurotoxicity induced by new psychoactive stimulants and psychedelics". Exp Neurol. 343: 113778. doi:10.1016/j.expneurol.2021.113778. PMID 34090893.
- ^ Marona-Lewicka D, Nichols DE (December 1997). "The Effect of Selective Serotonin Releasing Agents in the Chronic Mild Stress Model of Depression in Rats". Stress. 2 (2): 91–100. doi:10.3109/10253899709014740. PMID 9787258.
- ^ Scorza C, Silveira R, Nichols DE, Reyes-Parada M (July 1999). "Effects of 5-HT-releasing agents on the extracellullar hippocampal 5-HT of rats. Implications for the development of novel antidepressants with a short onset of action". Neuropharmacology. 38 (7): 1055–1061. doi:10.1016/S0028-3908(99)00023-4. PMID 10428424. S2CID 13714807.
- ^ a b c d Johnson MP, Nichols DE (July 1991). "Combined administration of a non-neurotoxic 3,4-methylenedioxymethamphetamine analogue with amphetamine produces serotonin neurotoxicity in rats". Neuropharmacology. 30 (7): 819–822. doi:10.1016/0028-3908(91)90192-e. PMID 1717873.
- ^ a b c Johnson MP, Conarty PF, Nichols DE (July 1991). "monoamine releasing and uptake inhibition properties of 3,4-methylenedioxymethamphetamine and p-chloroamphetamine analogues". Eur J Pharmacol. 200 (1): 9–16. doi:10.1016/0014-2999(91)90659-e. PMID 1685125.
- ^ Nichols DE, Marona-Lewicka D, Huang X, Johnson MP (1993). "Novel serotonergic agents". Drug des Discov. 9 (3–4): 299–312. PMID 8400010.
- ^ a b c d e Halberstadt AL, Brandt SD, Walther D, Baumann MH (March 2019). "2-Aminoindan and its ring-substituted derivatives interact with plasma membrane monoamine transporters and α2-adrenergic receptors". Psychopharmacology (Berl). 236 (3): 989–999. doi:10.1007/s00213-019-05207-1. PMC 6848746. PMID 30904940.
- ^ a b Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS (January 2001). "Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin". Synapse. 39 (1): 32–41. doi:10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. PMID 11071707. S2CID 15573624.
- ^ Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW (March 2013). "Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive 'bath salts' products". Neuropsychopharmacology. 38 (4): 552–562. doi:10.1038/npp.2012.204. PMC 3572453. PMID 23072836.
- ^ a b c Blough B (July 2008). "Dopamine-releasing agents" (PDF). In Trudell ML, Izenwasser S (eds.). Dopamine Transporters: Chemistry, Biology and Pharmacology. Hoboken : Wiley. pp. 305–320. ISBN 978-0-470-11790-3. OCLC 181862653. OL 18589888W.
- ^ Glennon RA, Dukat M (2017). "Structure-Activity Relationships of Synthetic Cathinones". Neuropharmacology of New Psychoactive Substances (NPS). Current Topics in Behavioral Neurosciences. Vol. 32. pp. 19–47. doi:10.1007/7854_2016_41. ISBN 978-3-319-52442-9. PMC 5818155. PMID 27830576.
- ^ Partilla JS, Dersch CM, Baumann MH, Carroll FI, Rothman RB (1999). "Profiling CNS Stimulants with a High-Throughput Assay for Biogenic Amine Transporter Substractes". Problems of Drug Dependence 1999: Proceedings of the 61st Annual Scientific Meeting, The College on Problems of Drug Dependence, Inc (PDF). NIDA Res Monogr. Vol. 180. pp. 1–476 (252). PMID 11680410.
RESULTS. Methamphetamine and amphetamine potently released NE (IC50s = 14.3 and 7.0 nM) and DA (IC50s = 40.4 nM and 24.8 nM), and were much less potent releasers of 5-HT (IC50s = 740 nM and 1765 nM).
- ^ a b Setola V, Hufeisen SJ, Grande-Allen KJ, Vesely I, Glennon RA, Blough B, Rothman RB, Roth BL (June 2003). "3,4-methylenedioxymethamphetamine (MDMA, "Ecstasy") induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro". Molecular Pharmacology. 63 (6): 1223–1229. doi:10.1124/mol.63.6.1223. PMID 12761331. S2CID 839426.
- ^ a b Brandt SD, Walters HM, Partilla JS, Blough BE, Kavanagh PV, Baumann MH (December 2020). "The psychoactive aminoalkylbenzofuran derivatives, 5-APB and 6-APB, mimic the effects of 3,4-methylenedioxyamphetamine (MDA) on monoamine transmission in male rats". Psychopharmacology (Berl). 237 (12): 3703–3714. doi:10.1007/s00213-020-05648-z. PMC 7686291. PMID 32875347.
- ^ Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV (April 2012). "The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue". Neuropsychopharmacology. 37 (5): 1192–1203. doi:10.1038/npp.2011.304. PMC 3306880. PMID 22169943.
- ^ Marusich JA, Antonazzo KR, Blough BE, Brandt SD, Kavanagh PV, Partilla JS, Baumann MH (February 2016). "The new psychoactive substances 5-(2-aminopropyl)indole (5-IT) and 6-(2-aminopropyl)indole (6-IT) interact with monoamine transporters in brain tissue". Neuropharmacology. 101: 68–75. doi:10.1016/j.neuropharm.2015.09.004. PMC 4681602. PMID 26362361.