Neodymium(III) oxide
In today's world, Neodymium(III) oxide has acquired unprecedented relevance. Whether due to its impact on society, its influence in the business environment or its importance in people's daily lives, Neodymium(III) oxide has become a topic of constant debate and discussion. From its origins to its evolution today, Neodymium(III) oxide has been the subject of study and analysis by experts from different areas. In this article, we will explore different aspects related to Neodymium(III) oxide, from its implications in everyday life to its impact on the global level. Through a detailed and in-depth look, we seek to better understand the role Neodymium(III) oxide plays in our modern world and how it has become an integral part of our reality.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Neodymium(III) oxide
| |
| Other names
Neodymium oxide, Neodymium sesquioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.832 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Nd2O3 | |
| Molar mass | 336.48 g/mol |
| Appearance | light bluish gray hexagonal crystals |
| Density | 7.24 g/cm3 |
| Melting point | 2,233 °C (4,051 °F; 2,506 K) |
| Boiling point | 3,760 °C (6,800 °F; 4,030 K)[1] |
| .0003 g/100 mL (75 °C) | |
| +10,200.0·10−6 cm3/mol | |
| Structure | |
| Hexagonal, hP5 | |
| P-3m1, No. 164 | |
| Thermochemistry | |
Heat capacity (C)
|
111.3 J·mol−1·K−1[1] |
Std molar
entropy (S⦵298) |
158.6 J·mol−1·K−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−1807.9 kJ·mol−1 |
| Related compounds | |
Other anions
|
Neodymium(II) chloride Neodymium(III) chloride |
Other cations
|
Uranium(VI) oxide Praseodymium(III) oxide Promethium(III) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Neodymium(III) oxide or neodymium sesquioxide is the chemical compound composed of neodymium and oxygen with the formula Nd2O3. It forms very light grayish-blue hexagonal crystals.[1] The rare-earth mixture didymium, previously believed to be an element, partially consists of neodymium(III) oxide.[2]
Uses
Neodymium(III) oxide is used to dope glass, including sunglasses, to make solid-state lasers, and to color glasses and enamels.[3] Neodymium-doped glass turns purple due to the absorbance of yellow and green light, and is used in welding goggles.[4] Some neodymium-doped glass is dichroic; that is, it changes color depending on the lighting. One kind of glass named for the mineral alexandrite appears blue in sunlight and red in artificial light.[5] About 7000 tonnes of neodymium(III) oxide are produced worldwide each year. Neodymium(III) oxide is also used as a polymerization catalyst.[4]
Reactions
Neodymium(III) oxide is formed when neodymium(III) nitride or neodymium(III) hydroxide is roasted in air.[6]
Structure
Neodymium(III) oxide has a low-temperature trigonal A form in space group P3m1.[7] This structure type is favoured by the early lanthanides.[8][9] At higher temperatures it adopts two other forms, the hexagonal H form in space group P63/mmc and the cubic X form in Im3m. The high-temperature forms exhibit crystallographic disorder.[10][11]
| Packing | Neodymium coordination | Oxygen O1 coordination | Oxygen O2 coordination |
|---|---|---|---|

|

|
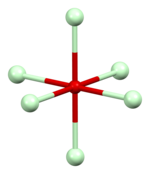
|

|
| A-M2O3 structure type | approximately capped octahedral | octahedral | approximately tetrahedral |
References
- ^ a b c Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 471, 552, ISBN 0-8493-0594-2
- ^ Brady, George Stuart; Clauser, Henry R.; Vaccari, John A. (2002), Materials Handbook (15 ed.), New York: McGraw-Hill Professional, p. 779, ISBN 978-0-07-136076-0, retrieved 2009-03-18
- ^ Eagleson, Mary (1994), Concise Encyclopedia of Chemistry, Springer, p. 680, ISBN 978-3-11-011451-5, retrieved 2009-03-18
- ^ a b Emsley, John (2003), Nature's Building Blocks, Oxford University Press, pp. 268–9, ISBN 978-0-19-850340-8, retrieved 2009-03-18
- ^ Bray, Charles (2001), Dictionary of Glass (2 ed.), University of Pennsylvania Press, p. 103, ISBN 978-0-8122-3619-4, retrieved 2009-03-18
- ^ Spencer, James Frederick (1919), The Metals of the Rare Earths, London: Longmans, Green, and Co, p. 115, retrieved 2009-03-18
- ^ D. Taylor (1984). "Thermal Expansion Data: III Sesquioxides, U2N3, with the corundum and the A-, B- and C-M2O3 structures". Trans. J. Br. Ceram. Soc. 83: 92–98.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 1238-1239. ISBN 978-0-08-037941-8.
- ^ A. F. Wells (1984). Structural Inorganic Chemistry (5th ed.). Oxford University Press. pp. 544–547.
- ^ Müller-Buschbaum, H. (1966). "Zur Struktur der A-Form der Sesquioxide der Seltenen Erden. II. Strukturuntersuchung an Nd2O3". Z. Anorg. Allg. Chem. 343 (1–2): 6–10. doi:10.1002/zaac.19663430103.
- ^ Aldebert, P.; Traverse, J. P. (1979). "Etude par diffraction neutronique des structures de haute temperature de La2O3 et Nd2O3". Mater. Res. Bull. 14 (3): 303–323. doi:10.1016/0025-5408(79)90095-3.