Sodium hyponitrite
Appearance move to sidebar hide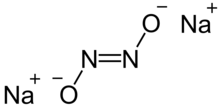
| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) |
|
| ChemSpider |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Na2N2O2 |
| Molar mass | 105.99 g/mol |
| Appearance | colorless crystals |
| Density | 2.466 g/cm3 |
| Melting point | 100 °C (212 °F; 373 K) |
| Boiling point | 335 °C (635 °F; 608 K) decomposes |
| Solubility in water | soluble |
| Solubility | insoluble in ethanol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Sodium hyponitrite is a solid ionic compound with formula Na
2N
2O
2 or (Na+
)22−.
There are cis and trans forms of the hyponitrite ion N
2O2−
2. The trans form is more common, but the cis form can be obtained too, and it is more reactive than the trans form.
Trans isomer
The trans isomer is colorless and soluble in water and insoluble in ethanol and ether.
Preparation
Sodium hyponitrite (trans) is conventionally prepared by reduction of sodium nitrite with sodium amalgam.
2 NaNO2 + 4 Na(Hg) + 2 H2O → Na2N2O2 + 4 NaOH + 4 HgSodium hyponitrite (trans) was prepared in 1927 by A. W. Scott by reacting alkyl nitrites, hydroxylammonium chloride, and sodium ethoxide
RONO + NH2OH + 2 EtONa → Na2N2O2 + ROH + 2 EtOHAn earlier method, published by D. Mendenhall in 1974, reacted gaseous nitric oxide (NO) with sodium metal in 1,2-dimethoxyethane, toluene, and benzophenone. The salt was then extracted with water. The method was later modified to use pyridine.
Other methods included oxidation of a concentrated solution of hydroxylamine with sodium nitrite in an alkaline medium; or electrolysis of sodium nitrite.
Hydrates
A variety of hydrates Na
2N
2O
2(H
2O)x of the trans isomer have been reported, with x including 2, 3.5, 4, 5, 6, 7, 8, and 9; but there is some dispute.
The hydration water seems to be just trapped in the crystal lattice rather than coordinated to the ions. The anhydrous substance can be obtained by drying the hydrates over phosphorus pentoxide and then heating them to 120 °C.
Reactions
Sodium hyponitrite (trans) in solution is decomposed by carbon dioxide CO
2 from air to form sodium carbonate.
Liquid N2O4 oxidises sodium hyponitrite (trans) to give sodium peroxohyponitrite Na2+
22−).
Cis isomer
The cis isomer of sodium hyponitrite is a white crystalline solid, insoluble in aprotic solvents, and (unlike the trans isomer) decomposed by water and other protic solvents.
Preparation
The cis isomer of can be prepared by passing nitric oxide (NO) through a solution of sodium metal in liquid ammonia at −50 °C.
The cis isomer was also obtained in 1996 by C. Feldmann and M. Jansen by heating sodium oxide Na
2O with 77 kPa of nitrous oxide N
2O (laughing gas) in a sealed tube at 360 °C for 2 hours. The two reagents combined to yield the cis hyponitrite quantitatively as white microcrystals.
Properties and reactions
The anhydrous cis salt is stable up to 325 °C, when it disproportionates to nitrogen and sodium orthonitrite:
3 Na2N
2O
2 → 2 Na
3O(NO
2) + 2 N
2
It is generally more reactive than the trans isomer.
See also
References
- ^ a b c d e Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
- ^ a b c d Claus Feldmann, Martin Jansen (1996), "cis-Sodium Hyponitrite - A New Preparative Route and a Crystal Structure Analysis". Angewandte Chemie International Edition in English, volume 35, issue 15, pages 1728–1730. doi:10.1002/anie.199617281
- ^ a b Trambaklal Mohanlal Oza, Rajnikant Hariprasad Thaker (1955), "The Thermal Decomposition of Silver Hyponitrite". Journal of the American Chemical society, volume 77, issue 19, pages 4976–4980. doi:10.1021/ja01624a007
- ^ a b A. W. Scott (1927), "Sodium Hyponitrite". J. Am. Chem. Soc., volume = 49, issue 4, pages = 986–987. doi:10.1021/ja01403a502
- ^ Addison, C. C.; Gamlen G. A.; Thompson, R. (1952). "70. The ultra-violet absorption spectra of sodium hyponitrite and sodium α-oxyhyponitrite : the analysis of mixtures with sodium nitrite and nitrate". J. Chem. Soc.: 338–345. doi:10.1039/jr9520000338.
- ^ Neumann, R. C., Jr. Bussey, R. J. (1970). "High pressure studies. V. Activation volumes for combination and diffusion of geminate tert-butoxy radicals". J. Am. Chem. Soc. 92 (8): 2440–2445. doi:10.1021/ja00711a039.{{cite journal}}: CS1 maint: multiple names: authors list (link)
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ a b Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 15: The group 15 elements". Inorganic Chemistry (3rd ed.). Pearson. p. 468. ISBN 978-0-13-175553-6.
- ^ G. David Mendenhall (1974), "Convenient synthesis of silver hyponitrite". Journal of the American Chemical society, volume 96, issue 15, page 5000. doi:10.1021/ja00822a054
- ^ Polydoropoulos, C. N. Chem. Ind. (London) 1963, 1686 and references therein.
- ^ James Riddick Partington and Chandulal Chhotalal Shah (1931), "Investigations on hyponitrites. Part I. Sodium hyponitrite: preparation and properties". Journal of the Chemical Society (Resumed), paper CCLXXXII, pages 2071-2080. doi:10.1039/JR9310002071
- ^ C.N. Polydoropoulos, S.D. Voliotis (1967), "Sodium hyponitrite hexahydrate". Journal of Inorganic and Nuclear Chemistry, volume 29, issue 12, pages 2899–2901. doi:10.1016/0022-1902(67)80121-0
- ^ a b c Gary L. Stucky, Jack L. Lambert, R. Dean Dragsdorf (1969), "The hydrates of sodium hyponitrite". Journal of Inorganic and Nuclear Chemistry, volume 31, issue 1, pages 29–32 doi:10.1016/0022-1902(69)80050-3
- ^ Charlotte N. Conner, Caroline E. Donald, Martin N. Hughes, Christina Sami (1989), "The molar absorptivity of sodium hyponitrite". Polyhedron, volume 8, issue 21, pages 2621-2622. doi:10.1016/S0277-5387(00)81166-3
- ^ M. N. Hughes and H. G. Nicklin (1969), "The action of dinitrogen tetroxide on sodium hyponitrite". Journal of the Chemical Society D: Chemical Communications, volume 1969, issue 2, page 80a. doi:10.1039/C2969000080A
| Sodium compounds | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inorganic |
| ||||||||||||||
| Organic | |||||||||||||||