Wagner–Meerwein rearrangement
In this article, we are going to analyze Wagner–Meerwein rearrangement in detail, exploring its different facets and characteristics to understand its impact in various contexts. From its origin to its relevance today, Wagner–Meerwein rearrangement has aroused notable interest and debate, becoming a topic of interest for experts and the general public. Along these lines, we will examine its historical evolution, its implications in contemporary society and the possible ramifications it has for the future. This article seeks to provide a comprehensive perspective on Wagner–Meerwein rearrangement, thus offering a solid starting point for those interested in delving into this complex and fascinating topic.
A Wagner–Meerwein rearrangement is a class of carbocation 1,2-rearrangement reactions in which a hydrogen, alkyl or aryl group migrates from one carbon to a neighboring carbon.[1] [2] They can be described as cationic -sigmatropic rearrangements, proceeding suprafacially and with stereochemical retention. As such, a Wagner–Meerwein shift is a thermally allowed pericyclic process with the Woodward-Hoffmann symbol . They are usually facile, and in many cases, they can take place at temperatures as low as –120 °C. The reaction is named after the Russian chemist Yegor Yegorovich Vagner; he had German origin and published in German journals as Georg Wagner; and Hans Meerwein.
Several reviews have been published.[3][4][5][6][7]
The rearrangement was first discovered in bicyclic terpenes for example the conversion of isoborneol to camphene:[8]

The story of the rearrangement reveals that many scientists were puzzled with this and related reactions and its close relationship to the discovery of carbocations as intermediates.[9]
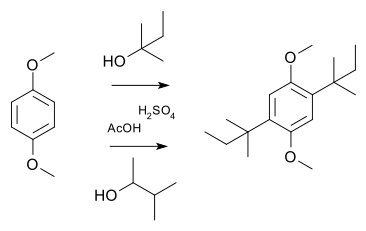
In a simple demonstration reaction of 1,4-dimethoxybenzene with either 2-methyl-2-butanol or 3-methyl-2-butanol in sulfuric acid and acetic acid yields the same disubstituted product,[10] the latter via a hydride shift of the cationic intermediate:
Currently, there are works relating to the use of skeletal rearrangement in the synthesis of bridged azaheterocycles. These data are summarized in [11]
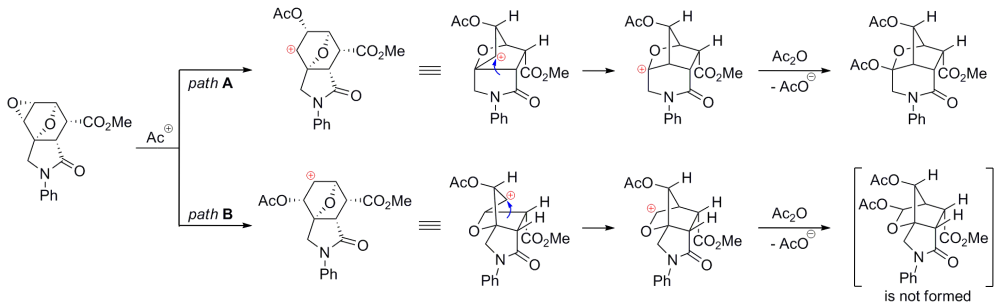
Plausible mechanisms of the Wagner–Meerwein rearrangement of diepoxyisoindoles:
The related Nametkin rearrangement, named after Sergey Namyotkin, involves the rearrangement of methyl groups in certain terpenes. In some cases the reaction type is also called a retropinacol rearrangement (see pinacol rearrangement).
References
- ^ Vagner, Ye. Ye. (Wagner, G.) (1899). "In "Protokol zasedaniya Otdeleniya Khimii R. F. Khimicheskago Obshchestva. 9-go sentyabrya 1899 goda [Minutes of the meeting of the Chemistry Section of the Russian Physical-Chemical Society. 9th September 1899]"". J. Russ. Phys. Chem. Soc. 31: 680–684.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hans Meerwein (1914). "Über den Reaktionsmechanismus der Umwandlung von Borneol in Camphen; [Dritte Mitteilung über Pinakolinumlagerungen.]". Justus Liebig's Annalen der Chemie. 405 (2): 129–175. doi:10.1002/jlac.19144050202.
- ^ Popp, F. D.; McEwen, W. E. (1958). "Polyphosphoric Acids As a Reagent in Organic Chemistry". Chem. Rev. 58 (2): 375. doi:10.1021/cr50020a004.
- ^ Cargill, Robert L.; Jackson, Thomas E.; Peet, Norton P.; Pond, David M. (1974). "Acid-catalyzed rearrangements of β,γ-unsaturated ketones". Acc. Chem. Res. 7 (4): 106–113. doi:10.1021/ar50076a002.
- ^ Olah, G. A. (1976). "Stable carbocations, 189. The σ-bridged 2-norbornyl cation and its significance to chemistry". Acc. Chem. Res. 9 (2): 41–52. doi:10.1021/ar50098a001.
- ^ Hogeveen, H.; Van Krutchten, E. M. G. A. (1979). "Wagner-meerwein rearrangements in long-lived polymethyl substituted bicycloheptadienyl cations". Top. Curr. Chem. Topics in Current Chemistry. 80: 89–124. doi:10.1007/BFb0050203. ISBN 3-540-09309-5.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hanson, J. R. (1991). "Wagner–Meerwein Rearrangements". Compr. Org. Synth. 3: 705–719. doi:10.1016/B978-0-08-052349-1.00077-9. ISBN 978-0-08-052349-1.
- ^ March, Jerry (1985). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.). New York: Wiley. ISBN 9780471854722. OCLC 642506595.
- ^ Birladeanu, L. (2000). "The Story of the Wagner-Meerwein Rearrangement". J. Chem. Educ. 77 (7): 858–863. Bibcode:2000JChEd..77..858B. doi:10.1021/ed077p858.
- ^ Polito, Victoria; Hamann, Christian S.; Rhile, Ian J. (2010). "Carbocation Rearrangement in an Electrophilic Aromatic Substitution Discovery Laboratory". Journal of Chemical Education. 87 (9): 969. Bibcode:2010JChEd..87..969P. doi:10.1021/ed9000238.
- ^ Zubkov, F. I.; Zaytsev, V. P.; Nikitina, E. V.; Khrustalev, V. N.; Gozun, S. V.; Boltukhina, E. V.; Varlamov, A. V. (2011). "Skeletal Wagner–Meerwein rearrangement of perhydro-3a,6;4,5-diepoxyisoindoles". Tetrahedron. 67 (47): 9148. doi:10.1016/j.tet.2011.09.099.