Peroxynitrite
In this article, we will explore Peroxynitrite in depth and its impact on our daily lives. From its origin to its contemporary relevance, we will analyze how Peroxynitrite has evolved over time and how it has affected different aspects of society. We will also examine the different perspectives and opinions related to Peroxynitrite, as well as its role in the current context. Through this comprehensive analysis, we hope to provide a complete and rich insight into Peroxynitrite, providing the reader with a deeper understanding of this topic.
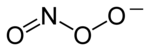 Chemical structure of the peroxynitrite anion
| |
| Names | |
|---|---|
| IUPAC name
Oxido nitrite
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| NO3− | |
| Molar mass | 62.005 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |

Peroxynitrite (sometimes called peroxonitrite) is an ion with the formula ONOO−. It is a structural isomer of nitrate, NO−
3
Preparation
Peroxynitrite can be prepared by the reaction of superoxide with nitric oxide:[1][2][3]
- NO + O−2 → NO(O2)−
It is prepared by the reaction of hydrogen peroxide with nitrite:[4]
- H2O2 + NO−
2 → ONOO− + H2O
Its presence is indicated by the absorbance at 302 nm (pH 12, ε302 = 1670 M−1 cm−1).
Reactions
Peroxynitrite is weakly basic with a pKa of ~6.8.
It is reactive toward DNA and proteins.
ONOO− reacts nucleophilically with carbon dioxide. In vivo, the concentration of carbon dioxide is about 1 mM, and its reaction with ONOO− occurs quickly. Thus, under physiological conditions, the reaction of ONOO− with carbon dioxide to form nitrosoperoxycarbonate (ONOOCO−
2) is by far the predominant pathway for ONOO−. ONOOCO−
2 homolyzes to form carbonate radical and nitrogen dioxide, again as a pair of caged radicals. Approximately 66% of the time, these two radicals recombine to form carbon dioxide and nitrate. The other 33% of the time, these two radicals escape the solvent cage and become free radicals. It is these radicals (carbonate radical and nitrogen dioxide) that are believed to cause peroxynitrite-related cellular damage.
Peroxynitrous acid
Its conjugate acid peroxynitrous acid is highly reactive, although peroxynitrite is stable in basic solutions.[5][6]
See also
References
- ^ Bohle, D. Scott; Sagan, Elisabeth S. (2004). "Tetramethylammonium Salts of Superoxide and Peroxynitrite". Inorganic Syntheses: 36. doi:10.1002/0471653683.ch1.
- ^ Pacher, P; Beckman, J. S; Liaudet, L (2007). "Nitric oxide and peroxynitrite in health and disease". Physiological Reviews. 87 (1): 315–424. doi:10.1152/physrev.00029.2006. PMC 2248324. PMID 17237348.
- ^ Szabó, C; Ischiropoulos, H; Radi, R (2007). "Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics". Nature Reviews Drug Discovery. 6 (8): 662–80. doi:10.1038/nrd2222. PMID 17667957.
- ^ Beckman, J. S; Koppenol, W. H (1996). "Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly". American Journal of Physiology. Cell Physiology. 271 (5 Pt 1): C1424–37. doi:10.1152/ajpcell.1996.271.5.C1424. PMID 8944624.
- ^ Holleman, A. F.; Wiberg, E. Inorganic Chemistry Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Koppenol, W. H (1998). "The chemistry of peroxynitrite, a biological toxin". Química Nova. 21 (3): 326–331. doi:10.1590/S0100-40421998000300014.