Tavilermide
This article will address the topic of Tavilermide from different perspectives with the aim of delving into its relevance and impact today. Tavilermide has captured the attention of various sectors due to its impact on society, the economy, politics and culture. Throughout the next few lines, its origins, evolution, challenges and opportunities, as well as its influence on social and technological change, will be analyzed. In addition, different studies and research that have shed light on Tavilermide and its relationship with other phenomena will be examined. In short, this article aims to offer a global and updated vision of Tavilermide, with the aim of contributing to the debate and reflection on this topic that is so relevant today.
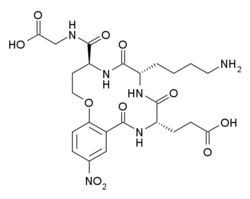 | |
| Clinical data | |
|---|---|
| Routes of administration | Eye drop |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H32N6O11 |
| Molar mass | 580.551 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tavilermide (INN) (developmental code name MIM-D3) is a selective, cyclic tripeptide partial agonist of TrkA. (this class of drugs is sometimes referred to as nerve growth factor (NGF) mimetics)[1][2][3] Tavilermide was first synthesized by Burgess and co-workers at Texas A&M University with the intention of producing TrkA agonists.[4][5] It is under development by Mimetogen Pharmaceuticals as an ophthalmic (eye drop) solution for the treatment of dry eyes, and is in phase III clinical trials for this indication. Tavilermide is currently being evaluated in two multi-center phase III clinical studies in the United States for the treatment of dry eye disease. Tavilermide is also in phase I clinical trials for the treatment of glaucoma; studies are ongoing.[6][7]
See also
References
- ^ Meerovitch K, Torkildsen G, Lonsdale J, Goldfarb H, Lama T, Cumberlidge G, Ousler GW (2013). "Safety and efficacy of MIM-D3 ophthalmic solutions in a randomized, placebo-controlled Phase 2 clinical trial in patients with dry eye". Clinical Ophthalmology. 7: 1275–1285. doi:10.2147/OPTH.S44688. PMC 3699314. PMID 23836957.
- ^ Jain P, Li R, Lama T, Saragovi HU, Cumberlidge G, Meerovitch K (October 2011). "An NGF mimetic, MIM-D3, stimulates conjunctival cell glycoconjugate secretion and demonstrates therapeutic efficacy in a rat model of dry eye". Experimental Eye Research. 93 (4): 503–512. doi:10.1016/j.exer.2011.06.014. PMID 21726552.
- ^ Vickers LA, Gupta PK (December 2015). "The Future of Dry Eye Treatment: A Glance into the Therapeutic Pipeline". Ophthalmology and Therapy. 4 (2): 69–78. doi:10.1007/s40123-015-0038-y. PMC 4675732. PMID 26289997.
- ^ Feng Y, Wang Z, Jin S, Burgess K (1998). "SNAr Cyclizations To Form Cyclic Peptidomimetics of β-Turns". Journal of the American Chemical Society. 120 (41): 10768–10769. doi:10.1021/ja981589t.
- ^ Feng Y, Burgess K (1999). "Solid-phase SNAr macrocyclizations to give turn-extended-turn peptidomimetics". Chemistry: A European Journal. 5 (11): 3261–3272. doi:10.1002/(SICI)1521-3765(19991105)5:11<3261::AID-CHEM3261>3.0.CO;2-H.
- ^ Chang EE, Goldberg JL (May 2012). "Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement". Ophthalmology. 119 (5): 979–986. doi:10.1016/j.ophtha.2011.11.003. PMC 3343191. PMID 22349567.
- ^ "Tavilermide". AdisInsight. Springer Nature Switzerland AG. Retrieved 18 August 2016.